
Meteorological&Communication

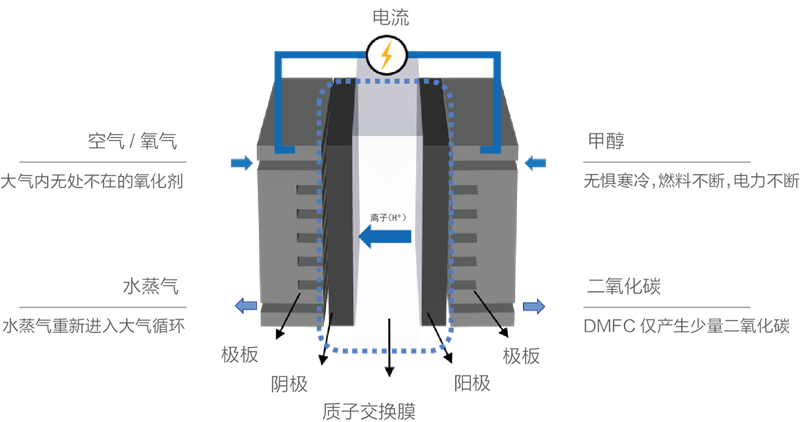
Methanol is oxidized by the anode catalyst to produce carbon dioxide, electrons and protons. Protons pass through the electrolyte membrane to reach the cathode region, and electrons work through the external circuit to reach the cathode region. At the cathode, protons and electrons react with oxygen with the aid of a catalyst whice the product is water.In general, the specific energy of methanol fuel is 500Wh/kg.

















